Sweet or Sour? A Gardener’s Guide to Soil pH
For most of us, we haven’t heard the term “pH” since high school chemistry. If you remember (and most of us don’t), pH stands for potential hydrogen and measures how acidic or basic something is. You may have heard that juice and coffee are acidic substances, and this is because they have a pH of below seven. Substances with a pH above seven, such as bleach, are termed basic or alkaline.
To help you remember, think of it like this: something basic is deemed sweet while something acidic is deemed sour.
Yet, pH isn’t only applicable to liquids. Did you know that your soil has a ph and that this number can determine which plants you grow, how well they grow, and how healthy your land is?
Soil pH: Alkaline vs. Acidic Soils
First, it should be noted that the optimal pH level for soil is 5.5 – 8 for topsoil and 4.8 – 8 for the sub-surface.
Alkaline soils are those whose pH level is above seven. They contain greater concentrations of sodium, calcium, and magnesium but fewer concentrations of phosphorous and micronutrients. Thus, they are less soluble than neutral and acidic soils.
Acidic soils are those whose pH level is less than seven. Unlike alkaline soils, they exhibit fewer concentrations of sodium, calcium, and magnesium and contain greater concentrations of manganese and aluminum, which can impair plant growth.
As you can see, both alkaline and acidic soils pose issues for plants. Soil alkalinity, however, is not hugely detrimental to plant growth. In fact, there are various plants that thrive in alkaline soils, such as:
- Asparagus
- Yams
- Cabbage
- Beets
- Cucumber
- Celery
- Cauliflower
- Gardenia
- Flox
- Lavender
- Hydrangea
Soil acidification, however, poses a serious problem not only to plants but to the agricultural sector as a whole.
Soil Acidification
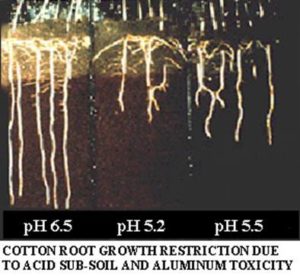
Acidic soil restricts a plant’s roots’ access to water and nutrients due to an increase in aluminum, which retards root growth. When roots cannot access nutrients (such as phosphorous, nitrogen, potassium, sulfur, calcium, and manganese), they begin to die.
Additionally, acidic soils can deplete colonies of microbial bacteria , especially rhizobia bacteria, which are responsible for breaking down organic matter for plant use.
Soil acidification is a large problem around the globe because it affects our crop production. Acidic soils can become unusable and degraded, forcing farmers to find new land on which to grow crops. This creates a cycle in which we continue to degrade the land without restoring the damaged soil.
So what’s the cause?
Conventional fertilizer use.
Toxic Conventional Fertilizers
Conventional fertilizers contain concentrations of ammonium nitrate, potassium nitrate, and calcium nitrate, which leave residual salts in the soil, leading to acidification.
Ammonium nitrate is one of the chief causes of soil acidification. When added as fertilizer, ammonium nitrate is converted into nitrate and hydrogen ions within the soil. If these ions are not absorbed by plants, the hydrogen ions within the soil will decrease the soil’s pH level and, thus, increase acidity.
Plants only absorb 50 percent of nitrogen from fertilizers, so when using conventional fertilizers, 50 percent will inevitably remain in the soil, resulting in two things:
- Soil acidification from the left-over hydrogen ions and
- Nitrous oxide (a greenhouse gas) production from microbes who consume leftover nitrate ions (you can read more about this here)
In order to raise soil pH levels and prevent soil acidification, farmers and gardeners can do one thing differently: invest in an eco-friendly fertilizer.
xVital for Soil pH
xVirity’s eco-friendly fertilizer is revolutionizing the way we grow plants.
xVital is a liquid fertilizer that contains only two ingredients: ionized water and nitrate. It is 100 percent chemical-free and salt-free; thus, it does not cause soil acidification.
In addition to this, xVital can be used to improve soil pH levels. Used as a pH downer and combined with either water or potassium carbonate (potash), xVital can effectively balance your soil’s pH.
If gardening trouble is beginning to make you feel a little sour, order a bottle of xVital today to make things just a little sweeter.
Didn’t Know Conventional Fertilizers were so Detrimental to the Earth? Read the Below Articles to Learn why you Should Switch to xVital
Fertilizer Produces Nitrous Oxide at Exponential Rates
Salt-Free Fertilizer Needed to Combat Increasing Salinity in Minnesota’s Freshwater Bodies
Nutrient Pollution Affecting Salt Marshes’ Ability to Sequester Carbon
Fertilizer Industry Causes 100x more Methane Released into Atmosphere than Previously Reported
Sources:

