Salt-Free Fertilizer Needed to Combat Water Salinization in Minnesota
In the state of Minnesota, the “Land of Ten Thousand Lakes,” 50 bodies of water have been classified as impaired and an additional 120 surface waters have been classified as high risk due to water salinization. (Retweet this!)
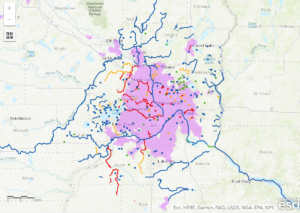
Figure 1: This map shows the bodies of water in Minnesota that have been classified as impaired in red and high risk in yellow. Purple indicates road density greater than or equal to 18 percent. Photo courtesy of the Minnesota Pollution Control Agency
This comes following water quality tests performed by the Minnesota Pollution Control Agency to measure the salinity levels of Minnesota’s freshwater bodies.
According to Brooke Asleson, Chloride Coordinator at MPCA, one teaspoon of salt per five gallons of water is enough to impair a freshwater ecosystem—and 50 bodies of water meet this threshold. Once the salt has dissolved in the water, there’s no way to extract it, so these bodies of water are permanently polluted.
MPCA officials have taken to educating the public on how to reduce salt pollution, sharing its Statewide Chloride Management Plan with the community.
So what is causing the salinity rise in Minnesota’s freshwater bodies? According to the MPCA, there are three contributing factors: road salt, water softener, and fertilizer.
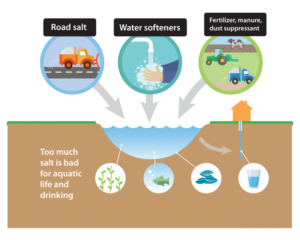
Figure 2: The three contributing factors to Minnesota’s salt crisis include road salt, water softeners, and fertilizer. Photo courtesy of the Minnesota Pollution Control Agency
A study conducted by the University of Minnesota revealed that 78 percent of road salt used in Twin Cities ends up in groundwater, lakes, and wetlands. Residents can reduce salt pollution by using less salt on their driveways and by recycling the salt and using it again. The MPCA also urges the state to use sand instead of ice on roads, parking lots, and sidewalks.
But why is the salinization of water so bad for the environment?
The effects of salinity in freshwater bodies include:
- Contaminated Drinking Water: a study found that chloride concentrations in 27 percent of monitoring wells in Twin Cities exceeded EPA drinking water guidelines. This could potentially make citizens ill.
- Loss of Aquatic Life: higher levels of salinity cause osmotic stress in freshwater organisms, as salt levels affect the way in which water moves in and out of these organisms’ cells. Thus, an increase in salinity can kill these organisms, reducing the biodiversity of freshwater ecosystems.
- Loss of Wildlife: deicing salt is toxic to various species of wildlife who drink from these freshwater bodies. In addition, animals, such as cats and dogs, can become ill if they consume or lick salty water.
- Soil Infertility and Erosion: increased salinity levels can make soil more susceptible to erosion and decrease its ability to retain water and nutrients.
- Impact on Plants: as with animals, increased salinity can cause osmotic stress in plants, stunting their growth and causing them to wilt. Furthermore, salt burns plant roots, which affects their ability to carry water.
Reducing the use of road salt and water softeners can help prevent the salinity of freshwater bodies—but what about fertilizer? As an essential component of agricultural practices, fertilizer use is difficult to regulate and reduce. Without inorganic fertilizer, plants wouldn’t receive the nutrients needed to grow. So how do we reduce the negative effects of fertilizer on water bodies?
xVital, xVirity’s liquid fertilizer, is salt-free; thus, it doesn’t contribute to soil and water salinization. Using xVital on your crops or garden eliminates water salinization via runoff, serving as an excellent solution for Minnesota’s salt crisis. Furthermore, it grows healthier plants than conventional fertilizers, increases plants’ ability to obtain and store water, and supports healthy soil.
By using xVital on your crops or garden, switching to sand rather than deicing salt, and reducing and eliminating the use of water softeners, Minnesotans can combat their salt crisis. Check out our About page for more information.
Sources:
Low Water and High Salinity: the Effects of Climate Change and Water Abstraction on Lake Ecosystems



